Biofilm-Disrupting Botanical Blends: A Strategic Guide to Addressing Resilient Gut Pathogens
Introduction
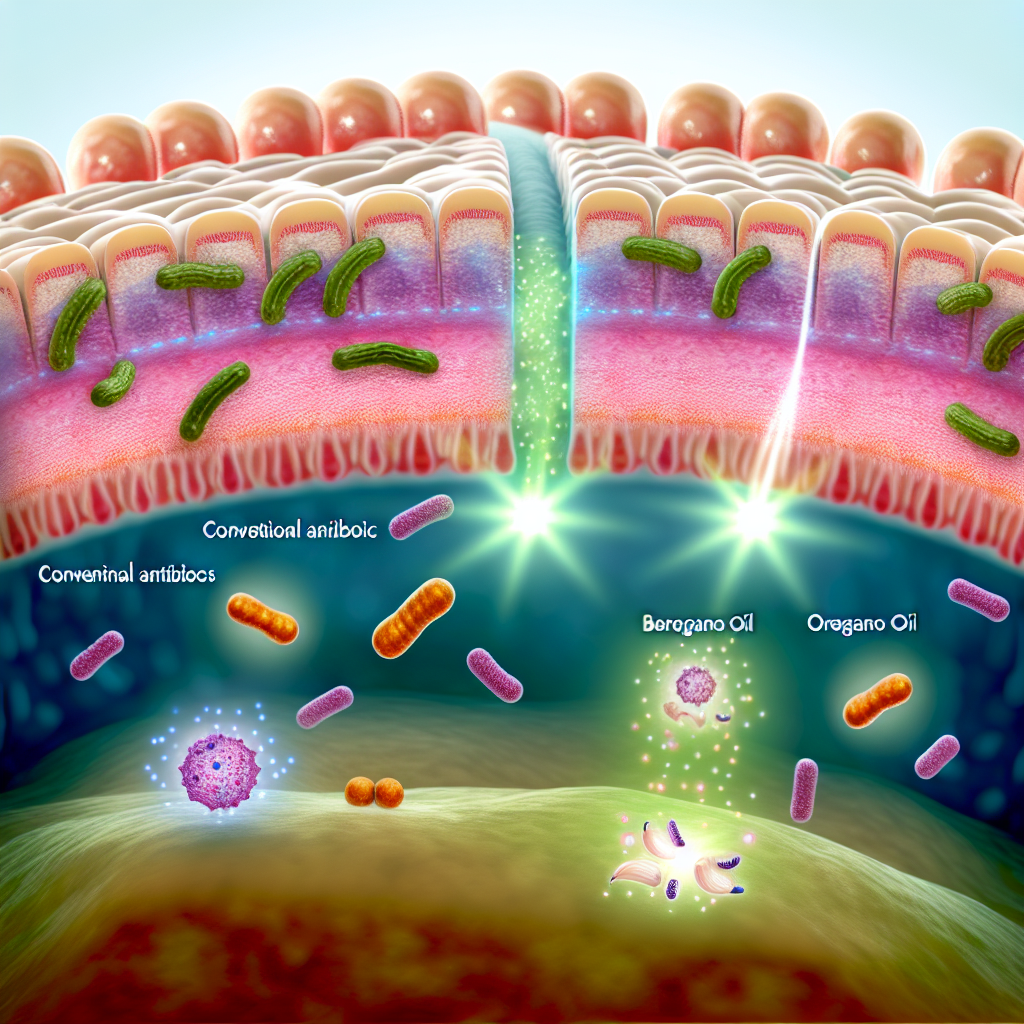
Maintaining a healthy gut microbiome is essential not just for digestion, but also for immunity, mental health, and chronic disease prevention. One of the lesser-known challenges in sustaining optimal gut health is the presence of biofilms formed by pathogenic microbes. These complex, slimy structures are composed of bacteria and extracellular polymers that create a protective barrier. This defense mechanism allows harmful microbes to evade immune detection and resist antibiotic or herbal treatments.
For individuals experiencing recurring gastrointestinal disturbances—such as bloating, persistent infections, irritable bowel syndrome (IBS), or leaky gut syndrome—hidden microbial biofilms may be a foundational contributor. Pathogens like Candida albicans, Escherichia coli (E. coli), and Clostridium difficile thrive in these environments, securing themselves within biofilms that can be 100 to 1,000 times more resistant to antimicrobials and the immune system.
Conventional treatments, especially chronic antibiotic use, often fail to eliminate them and may lead to an imbalanced microbiome and increased antibiotic resistance. Consequently, a rising interest in natural, holistic strategies has led researchers to explore botanicals that can effectively dismantle biofilms without harming beneficial gut flora.
Among these promising natural therapies are berberine, oregano oil, garlic extract (allicin), and cranberry polysaccharides. These compounds weaken the biofilm matrix and increase microbial vulnerability to immune clearance and therapeutic agents.
Integrative and functional medicine professionals now widely regard biofilm-disrupting botanical blends as key elements of comprehensive gut healing protocols. These combinations work synergistically to dismantle harmful fortifications while preserving the body’s symbiotic microbial environment.
In this guide, we explore current research and clinical applications behind these botanical agents, review effective blend formulations, and outline evidence-based practices for incorporating them into a healthy gut regimen.
Features and Supporting Scientific Research
Recent years have seen a surge in both medical and scientific interest regarding biofilm disruption and its significance in chronic disease. Biofilms are now recognized as culprits in various persistent infections, especially throughout the gastrointestinal tract.
One of the most studied anti-biofilm agents is berberine, an alkaloid derived from plants including Goldenseal (Hydrastis canadensis) and Berberis species. Investigations have shown its ability to obstruct bacterial communication systems such as quorum sensing, which bacteria use to coordinate biofilm construction. A 2019 study in Frontiers in Microbiology demonstrated berberine’s impressive ability to reduce adhesion and disrupt E. coli and Pseudomonas aeruginosa biofilms, increasing their susceptibility to antimicrobials ([Berberine and E. coli biofilms](https://www.frontiersin.org/articles/10.3389/fmicb.2019.00761/full)).
Allicin, the active sulfur compound in garlic (Allium sativum), has also been validated as a strong biofilm disruptor. It interrupts fungal and bacterial biofilms and inhibits Candida species, which are frequently associated with gut imbalances such as SIBO and candidiasis. Research in the Journal of Applied Microbiology supports the use of allicin for breaking down Candida biofilms ([Allicin against Candida Biofilms](https://sfamjournals.onlinelibrary.wiley.com/doi/10.1111/jam.14271)).
Essential oils have also demonstrated biofilm-fighting properties. Oregano oil contains antibacterial compounds such as carvacrol and thymol, which effectively inhibit biofilm development. A study published in Microbial Pathogenesis (2020) showed oregano oil’s ability to reduce biofilms formed by Staphylococcus aureus and restore the impact of antibiotics when used together ([Oregano Oil and Staphylococcus aureus Biofilms](https://www.sciencedirect.com/science/article/abs/pii/S0882401020308857)).
Cranberry extract contains D-mannose and proanthocyanidins, which interfere with bacterial adhesion—a critical first phase in biofilm formation. While commonly used for urinary tract health, its adhesion-preventing properties can also benefit gastrointestinal biofilms and protect mucosal lining integrity ([Cranberry Extract and Anti-Adhesion](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8907179/)).
In clinical applications, these botanicals are often part of synergistic protocols that combine antimicrobial herbs with systemic enzymes like nattokinase and serrapeptase. These enzymes function to degrade extracellular matrix proteins within biofilms, further enhancing pathogen exposure and clearance. By softening the biofilm’s structure, these enzymes allow botanical antimicrobials to better penetrate and neutralize embedded microbes.
When administered as part of a personalized treatment plan—often under the care of holistic or functional practitioners—biofilm-disrupting blends promote improvements in nutrient absorption, inflammation control, and resolution of long-standing digestive symptoms.
Conclusion
Biofilms represent a formidable obstacle in the journey toward optimal gut health. Their tenacious structure allows pathogens to thrive, even in the face of antibiotics and immune defenses. Traditional approaches are often ineffective, leaving many to suffer persistent gut issues.
Biofilm-disrupting botanical blends offer a targeted, nature-based solution. With growing empirical support from laboratory and clinical studies, compounds such as berberine, allicin, oregano oil, and cranberry extract are emerging as trusted tools in holistic digestive protocols. When used alongside supportive enzymes and lifestyle interventions, these blends strike at the core of biofilms, offering a sustainable path to restoring digestive resilience and overall health.
Concise Summary
Biofilms are protective layers formed by harmful microbes in the gut, shielding them from the immune system and treatments. These hidden structures are often involved in chronic gut issues like IBS and SIBO. Natural compounds such as berberine, garlic-derived allicin, oregano oil, and cranberry extract have shown powerful biofilm-disrupting properties. Used together in botanical blends—sometimes with enzymes like serrapeptase—they can dismantle biofilms, making pathogens easier to eliminate. Evidence supports their effectiveness and safety, making them valuable tools in restoring gut health. Personalized use under professional guidance is recommended for best outcomes.
References
– [Frontiers in Microbiology (2019) — Berberine and E. coli Biofilms](https://www.frontiersin.org/articles/10.3389/fmicb.2019.00761/full)
– [Journal of Applied Microbiology — Allicin against Candida Biofilms](https://sfamjournals.onlinelibrary.wiley.com/doi/10.1111/jam.14271)
– [Microbial Pathogenesis (2020) — Oregano Oil and S. aureus Biofilms](https://www.sciencedirect.com/science/article/abs/pii/S0882401020308857)
– [NIH (2022) — Cranberry Extract and Anti-Adhesion Properties](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8907179/)
– [Gut Microbes (2020) — Biofilm Contributions to Chronic Gastrointestinal Issues](https://www.tandfonline.com/doi/full/10.1080/19490976.2020.1735698)

Dominic E. is a passionate filmmaker navigating the exciting intersection of art and science. By day, he delves into the complexities of the human body as a full-time medical writer, meticulously translating intricate medical concepts into accessible and engaging narratives. By night, he explores the boundless realm of cinematic storytelling, crafting narratives that evoke emotion and challenge perspectives.
Film Student and Full-time Medical Writer for ContentVendor.com